What are Atoms?
Atoms are the smallest units of an element that still retain the properties of that element. They are like tiny building blocks that combine in various ways to form all the substances we see around us. From the air we breathe to the water we drink, from the food we eat to the stars we see in the sky – everything is made up of atoms.
Why are Atoms Important?
Atoms are important because they form the basis of all matter. By understanding atoms, we can understand the structure and properties of different materials. For instance, why is gold so shiny? Why is helium lighter than air? Why does sodium react so violently with water? The answers to all these questions lie in the structure and behavior of atoms.
The Nucleus
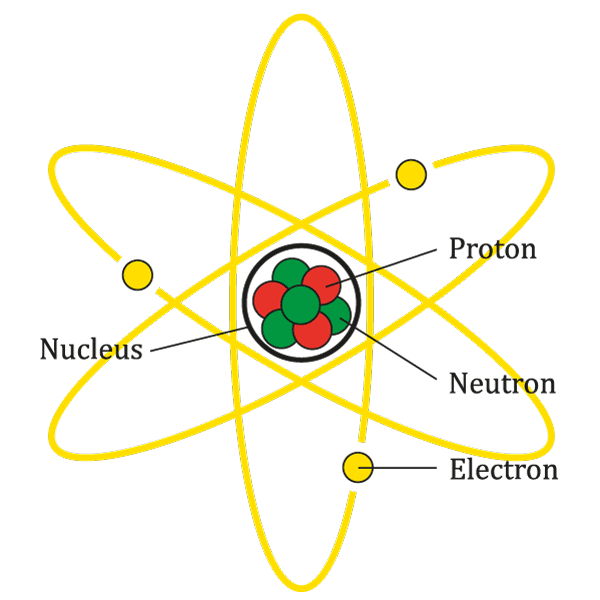
At the center of each atom is a dense core called the nucleus. Despite being incredibly tiny, the nucleus contains almost all of an atom’s mass. This is because the nucleus is home to two types of particles: protons and neutrons.
- Protons: Protons are positively charged particles found within the nucleus. The number of protons in an atom determines what element it is. For example, an atom with one proton is hydrogen, an atom with two protons is helium, and so on.
- Neutrons: Neutrons are particles that have no charge, hence the name ‘neutron’, which means ‘neutral’. They contribute to the mass of an atom and help to hold the nucleus together by counteracting the positive charge of the protons.
Electrons
Electrons are tiny particles that orbit the nucleus, similar to how planets orbit the sun. They carry a negative charge, which balances the positive charge of the protons in the nucleus. Electrons are involved in chemical reactions and the formation of chemical bonds. They also determine the electrical and thermal conductivity of materials.
The arrangement of electrons in an atom can change, moving to higher or lower energy levels or even jumping from one atom to another. These changes can result in the absorption or emission of light, leading to the colors we see in fireworks, neon lights, and even the rainbow!
Atomic Adventures
In a nutshell, atoms are the tiny bits that make up everything we see and touch, from the air to the stars. They’re made of protons, neutrons, and electrons. Understanding atoms helps us know why things in our world act the way they do.